Unlocking the Mysteries of Mycotoxin Levels in Feeds
One of history’s enduring quotes originates from the philosopher Aristotle, who articulated, “The whole is greater than the sum of its parts.” Today, we apply this wisdom to agriculture, specifically in understanding the cumulative effect of mycotoxins on livestock health and productivity.
We explore the effect of mycotoxin levels in livestock feeds and the significance of multiple mycotoxins in tandem, uncovering the holistic impact of these toxins on livestock well-being and performance. Also, we investigate historical research documenting threshold levels of concern for individual mycotoxins across various animal species. However, there is a notable gap in research exploring the combined effects of multiple different mycotoxins present in livestock diets.
The Cumulative Effect of Stressors
Producers want to understand the “safe” threshold of mycotoxin levels in livestock feed and consult various available guides. However, the speed at which mycotoxins become toxic is often contingent upon several factors, including environmental conditions, genetics, nutritional factors, and other stressors such as disease prevalence, heat, or overcrowding. Moreover, the presence of other mycotoxins in the diet can exacerbate these effects. These factors collectively influence the clinical expression of mycotoxicosis, adversely affecting weight gain, feed efficiency, production, and reproductive capabilities.
Under most normal production conditions, more than one mycotoxin is present in an animal’s ration. Toxin-producing fungi often generate more than one mycotoxin. This process can contaminate a single feed ingredient, or several different toxins can be blended into a diet from several different ingredients that make up finished feed. Furthermore, animals can encounter toxins from other sources, causing additional stress. These would include endo and exotoxins from bacteria, viral toxins, or toxins from the environment, both synthetic and natural.
Mycotoxin Interactions
Most controlled research uses dietary mycotoxin concentrations much higher than those shown to affect field conditions. The reason for using these higher concentrations is to achieve a significant challenge response in research conditions. As a result, the combined effects of multiple dietary mycotoxins in research may sometimes appear less than cumulative. This discrepancy could arise because of the severity of the negative effects induced by each mycotoxin, preventing the typical response from the second mycotoxin from occurring simultaneously in the animal.
The cumulative effects of multiple mycotoxins can manifest in various ways:
- Additive effects, where each mycotoxin independently decreases performance by the same amount as it would if present in the diet alone;
- Synergistic effects, where mycotoxins collaborate to exacerbate negative outcomes beyond their individual impacts; and rarely,
- Antagonistic effects, where one mycotoxin diminishes the activity of another, although this is not to be expected as supported by various research.
In a 2003 mycotoxin report by the Council for Agricultural Science and Technology there was a review of 33 papers where researchers reported the effects of multiple mycotoxins fed concurrently throughout production. In this review, only one was believed to have had an antagonistic response.
An interesting occurrence, often not discussed, occurs when multiple mycotoxins demonstrate potentiative effects. According to the CAST report, “Potentiative effects occur when one mycotoxin does not cause a toxic effect on a certain organ or system, but then fed with another mycotoxin it makes the latter much more toxic.”
What requires consideration and management is the overall toxin exposure. Achieving control over the disease is not dependent on attaining 100% control of any given toxin. Instead, it can be achieved by reducing a portion of the toxin exposure so that the total exposure is controlled.
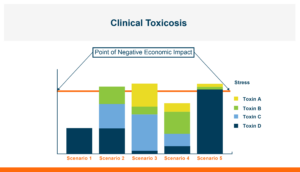
Figure 1. Control of toxicosis is not dependent on 100% control of any given toxin but can be attained by reduction of part of the toxin exposure such that the total exposure is dropped below the point that production is economically impacted.
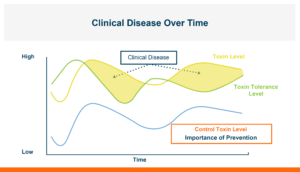
Figure 2. Multiple sources of stress in an animal’s life will change the tolerance level it has for toxins at different times. If you can decrease the toxin concentration that is entering the body from the gastrointestinal tract you can minimize the amount of damage it can do.
The animal’s ability to tolerate total mycotoxins can fluctuate over time due to varying levels of additional stressors impacting the animal.
These stressors encompass a wide range of factors including disease challenges, rapid growth, onset of egg production, weaning, pregnancy or nursing, exposure to other mycotoxins, or toxins in the diet, among others. In addition, the total concentration of mycotoxins in the diet will also change over time. Clinical disease becomes evident when the total mycotoxin level surpasses the animal’s tolerance threshold. To optimize performance, it is essential to both minimize animal stress and mitigate toxin exposure.
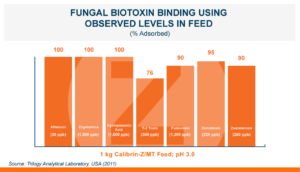
Calibrin®-Z: The Broadest Spectrum for Biotoxin Control
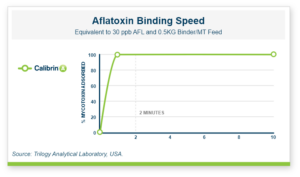
Calibrin®-Z, available in select international markets, is proven to adsorb a broad range of bacterial and fungal toxins that negatively impact livestock. It is supported by years of research at universities, research organizations, and on-farm use around the world.
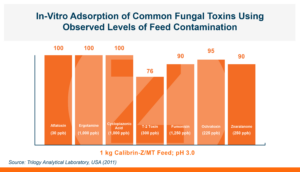
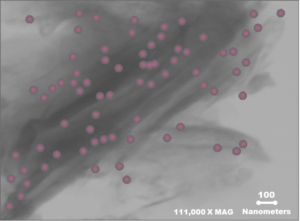
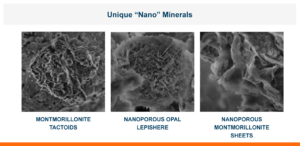
Calibrin-Z is not a traditional clay mineral. Amlan® International sources this unique, natural mineral in the U.S. and it is composed of calcium montmorillonite with high-capacity opal-lepispheres. It is a type of phyllosilicate constructed of nano-scale layers, providing an extensive surface area. Through proprietary thermal processing, Calibrin-Z is optimized to effectively bind the broadest range of biotoxins, including fungal and bacterial toxins. Selectively sourced for its exceptional binding capability, this mineral targets both polar toxins like aflatoxin and significant non-polar toxins such as zearalenone.
Calibrin-Z: A Multiple Toxin Solution
In the last year, numerous research projects have been undertaken to challenge and document the effectiveness of Calibrin-Z in mitigating the effects of multiple mycotoxins in the feed.
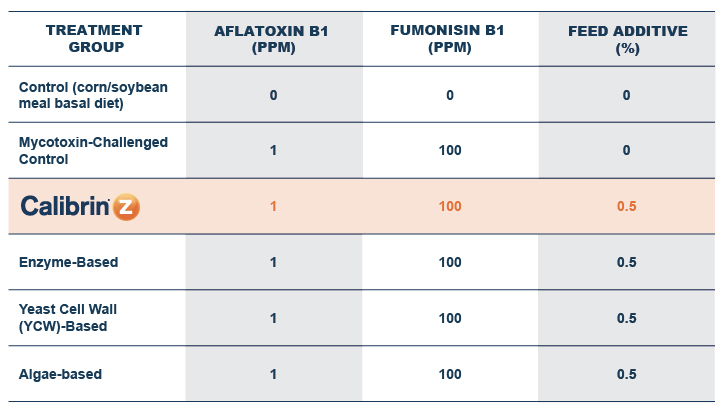
These studies encompass a variety of approaches, ranging from a side-by-side comparison of high levels of a singular toxin, a combination of multiple toxins at lower levels, and a study that evaluated the effects of low levels of multiple toxins (all below a normal company rejection level for each toxin).
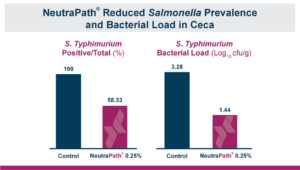
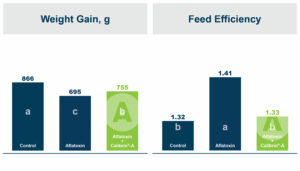
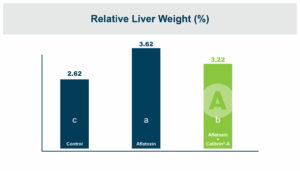
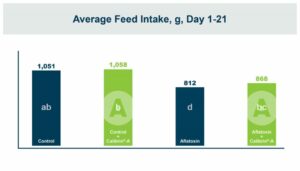
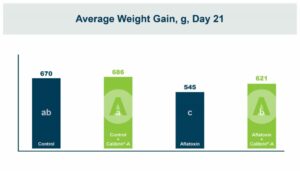
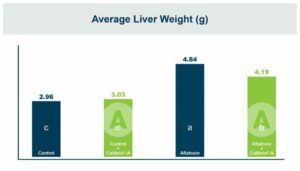
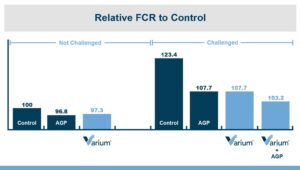
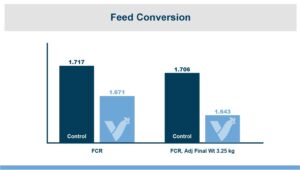
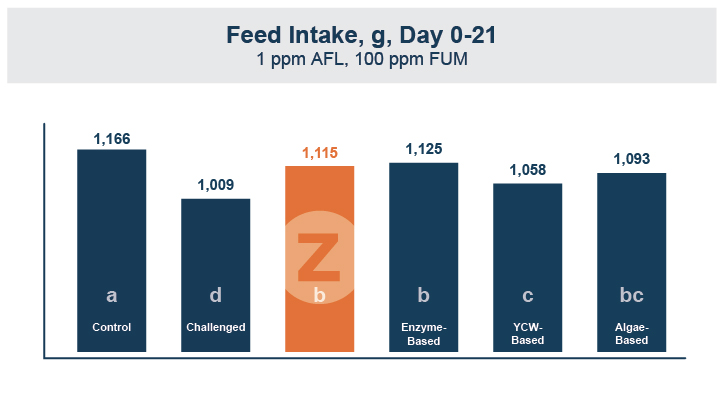
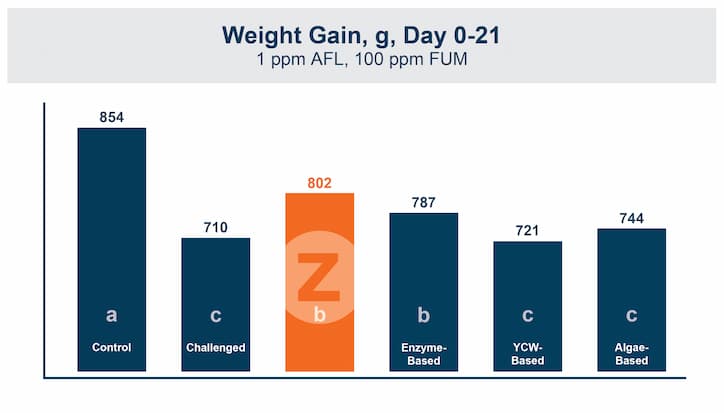
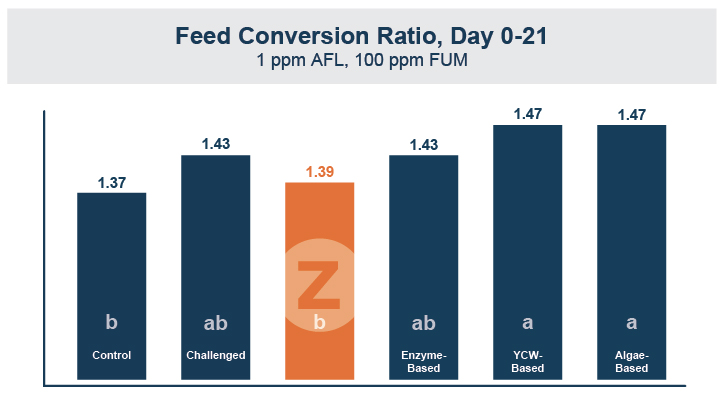
In the first study, a comparison was made between broiler chickens fed a higher concentration of T-2 toxin (2 ppm) and broilers fed a combination of multiple mycotoxins consisting of T-2 (1 ppm), Aflatoxin (1.8 ppm), and Fumonisin (50 ppm). The treatment groups in the trial included an unchallenged control group, a challenged control group for both the singular toxin challenge and the multiple toxin challenge, and challenge groups with Calibrin-Z included in the diet for both the single toxin and the multiple toxin challenges. Due to the high concentrations of mycotoxins in this study, Calibrin-Z was added at an inclusion rate of 0.5%. In all traits measured (BW, FCR, Relative Liver Weight, and Villus Height) the negative effect on performance was greater for the multiple mycotoxin challenge as opposed to the higher single mycotoxin challenge. When Calibrin-Z was incorporated into the diet, the result was a statistically significant improvement in performance vs. the challenged control for body weight (63g), relative liver weight, and villus height, and a numerically significant (6 points) improvement in FCR under multiple mycotoxin challenge.. For the single high mycotoxin challenge, the birds fed Calibrin-Z improved in every KPI with BW and FCR performing even numerically better than the unchallenged control.
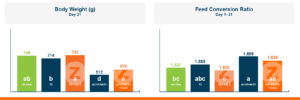
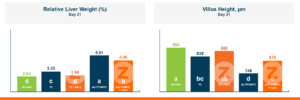
While it is anticipated to observe performance improvements when employing a high-quality toxin binder like Calibrin-Z, in diets containing elevated levels of both single and multiple mycotoxins, the initial query raised in this article remains pertinent: When individual toxin levels are exceedingly low, can they still adversely impact performance? And if so, can a toxin binder improve performance with low levels of toxins enough to offset the cost of inclusion in the diet?
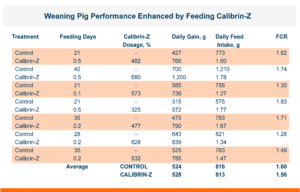
To test this, a replicated pen study was run that did not spike the broiler diet with mycotoxins, but rather tested the ingredients to document the level of mycotoxins naturally present. The corn was found to contain naturally occurring Fumonisin, T-2, and DON at levels significantly below what is documented as “Levels of Concern” in poultry.
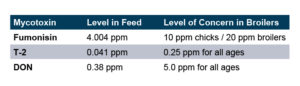
The trial contained 8 replicate pens of 23 male broilers for each treatment and compared a control group to a group fed the same diet with an inclusion of 1 KG/MT Calibrin-Z. As demonstrated in the table below, even when individual mycotoxin levels in the feed are extremely low, there is a synergistic effect that adversely impacts economically significant KPIs. However, incorporating a standard inclusion of Calibrin-Z into the feed improved performance.
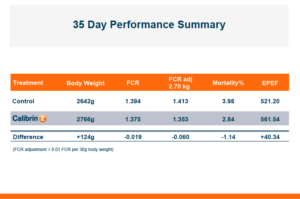
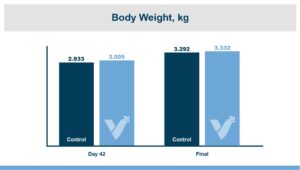
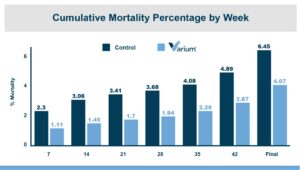
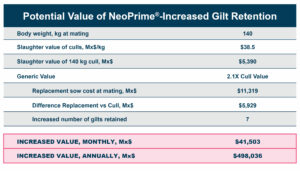
While the numerical improvements of approximately two days of growth (60 g/Day), a 6-point adjusted FCR, and a 1.14% increase in livability are certainly notable, what is the economic impact on live cost? If we assume a standard feed cost of $350/ton, which is common with the current grain prices, and then include the cost of the standard 1 kg/MT dosage of Calibrin-Z, these differences in KPI performance translate to a live cost advantage of just over $280,000 annually for every 100,000 broilers processed per week. Therefore, for a 1 million broiler per week operation producing 2.75 kg (approximately 6 lb.) broilers, this would result in a live cost savings based on enhanced performance of approximately $2.8 million annually.

As previously mentioned, much of the research, including the initial study cited, is conducted with mycotoxin levels significantly elevated. While this approach effectively demonstrates the negative impact of mycotoxins on performance and highlights the benefits of using a toxin binder like Calibrin-Z, it does not accurately reflect the levels typically encountered in real-world scenarios. Consequently, many producers opt to forego a toxin binder in their day-to-day feed formulations and only consider adding a binder during specific times of year when higher mycotoxin levels are anticipated.
However, this second study clearly demonstrates the synergistic interaction between extremely low levels that are under the “acceptable” threshold of mycotoxins and the significant economic advantages of incorporating a proven toxin binder, like Calibrin-Z, into the diet to enhance a producer’s bottom line.
To start a trial, visit Amlan.com
© 2024 Amlan International. All Rights Reserved. Product availability may vary by country, associated claims do not constitute medical claims and may differ based on government requirements.