Proprietary mineral technology is the foundation for Amlan’s innovative value-added products for animal protein producers. In this article, we take an in-depth look into the mineral technology used in our all-natural feed additive Calibrin®-Z and its unique properties that are the Amlan difference.
Consistent, Controlled Mineral Supply
The physical and chemical properties of a mineral can differ depending on where it is mined. That is why — to ensure consistent quality — Amlan only uses a single-source mineral in our products. Amlan is vertically integrated as the animal health business of Oil-Dri® Corporation of America, allowing Amlan and Oil-Dri to control every step of the production process and reliably deliver safe, high-quality products.
Calibrin-Z: Our All-Natural Broad-Spectrum Biotoxin Control Product
Calibrin-Z protects poultry and livestock health and performance by binding intestinal pathogens, bacterial exotoxins and endotoxins and polar and nonpolar mycotoxins. It is composed of a single ingredient — our proprietary mineral technology, thermally processed to create the specific physical and chemical properties that give Calibrin-Z its powerful mode of action.
A Network of Interconnected Pores
The distinctive properties of Calibrin-Z include a high surface area and extensive porosity. More than 99% of Calibrin-Z’s total surface area is internal due to the product’s structural properties. This means that targeted molecules can migrate via interconnected networks of capillary channels towards internal binding sites. These physical features provide Calibrin-Z with a high adsorption capacity for binding a broad range of mycotoxins, bacterial pathogens and their toxins.
Layers Within Layers
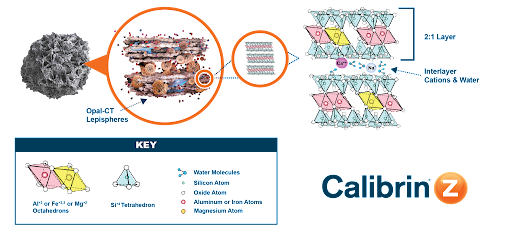
The mineral in Calibrin-Z is a particular type of phyllosilicate (“phyllo” meaning sheet) and is primarily calcium montmorillonite with amorphous opal-CT lepispheres and other minor and trace minerals.
Phyllosilicates consist of silicon, oxygen, magnesium and water molecules, and either aluminum or iron atoms. The aluminum, iron or magnesium atoms form octahedron structures, whereas the silicon forms tetrahedrons. These formations give the mineral a nano-scale structure of a 2:1 layer of octahedrons between tetrahedrons. Between the 2:1 layers are interlayers of water molecules and cations (Figure 1). Various positively charged sites in the mineral structure — interlayer cations and broken edge octahedral units — provide the adsorption sites.
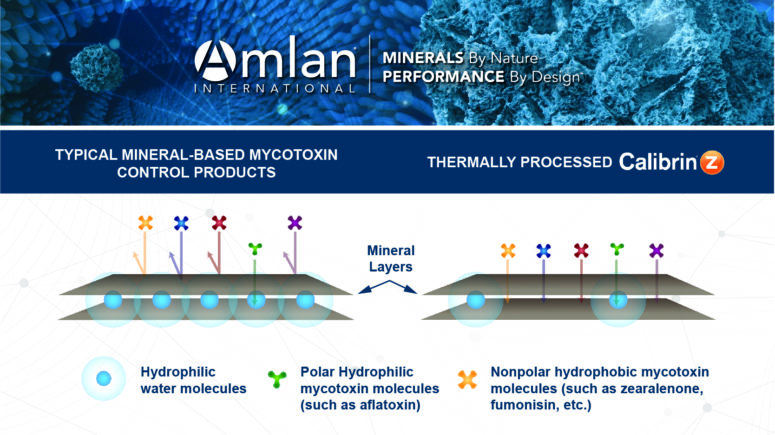
Proprietary Thermal Processing
Typical montmorillonites have water molecules between the mineral layers that make the pores and surfaces hydrophilic for adsorbing hydrophilic (polar) molecules (e.g., aflatoxins) but do not bind hydrophobic (nonpolar) molecules (e.g., zearalenone and fumonisin). However, the montmorillonite used in Calibrin-Z undergoes proprietary thermal processing that uses an optimized temperature and time to allow adsorption of hydrophilic and hydrophobic toxins (Figure 2).
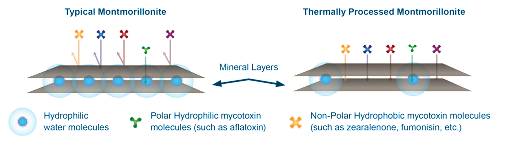
Thermal processing eliminates most of the water molecules from the mineral in Calibrin-Z, making it more hydrophobic. The process is carefully controlled since excessive heat that completely dries the mineral — removal of the interlayer water molecules — would destroy Calibrin-Z’s binding capabilities. The naturally occurring opal-CT lepispheres help maintain the layered sheet structure of the mineral during processing and provide Calibrin-Z’s high binding capacity (Figure 3). Amlan’s proprietary processing method also avoids the use of harmful chemicals typically used by other companies preserving a natural composition.

A Variety of Binding Mechanisms
Calibrin-Z’s binding forces include hydrophobic interactions, chelation, electrostatic attractions, hydrogen bonding and van der Waals forces. Thermal processing allows an interaction between both polar hydrophilic molecules and non-polar hydrophobic molecules and the inter-mineral layer. This is the method used to adsorb mycotoxins to Calibrin-Z.
Bacterial exotoxin binding to Calibrin-Z occurs through molecular ion exchange mechanisms. For example, a part of the Clostridium perfringens alpha-toxin electrostatically anchors (tethers) to either the positively charged broken-edge sites (exposed alumina octahedra) or the positively charged interlayer cations of Calibrin-Z.
Molecular conformation change mechanisms are also possible binding methods. Large exotoxins can distort their molecular structures or conformations to adsorb themselves onto macro-surfaces within the pore spaces.
Compatible With Nutrient Availability
While Calibrin-Z excels at binding biotoxins, its binding abilities do not interfere with the absorption of important nutrients in the diet. It is possible that some minor quantity of nutrients could temporarily be absorbed into Calibrin-Z’s pores. However, this is via weak thermodynamic and kinetic interactions that are readily reversible. Therefore, nutrients can travel in to and out of Calibrin-Z particles based on concentration gradients in the gastrointestinal tract.
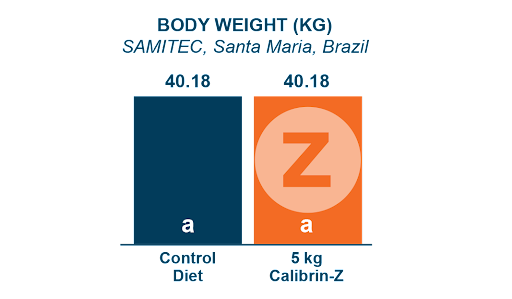
A 42-day swine study conducted by SAMITEC in Brazil, examined the performance of pigs fed a common basal diet (Control) and Calibrin-Z included at 5 kg/MT, a level that is 10 times the recommended dose. Even at this very high inclusion rate, Calibrin-Z had no adverse effects on nutrient availability, supporting equivalent weight gain and feed conversion.
The proprietary mineral technology used in Calibrin-Z is what sets it apart from other companies’ mineral-based products. For more information about Calibrin-Z and how it can help protect your animals from the deleterious effects of biotoxins, contact us at info@amlan.com.