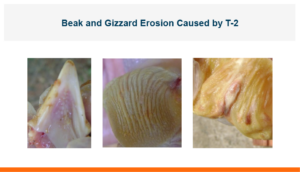
Poultry producers mostly know T-2 toxin by the horrific lesions seen on the beaks of poultry. The fast-acting T-2 toxin has a major impact on the growth and performance of poultry and livestock. Luckily it is not the most common trichothecenes toxin produced by Fusarium molds, deoxynivalenol would fill that spot, but T-2 is considered the most toxic of the trichothecenes. This poison can be inhaled or adsorbed through the skin or the gastro-intestinal tract and causes multiple problems in poultry and livestock. A short list of problems includes decreased gain and feed efficiency, decreased egg production and hatchability, decreased immune function, and increased mortality. It has been shown to have a synergistic negative effect with other mycotoxins in the diet or when administered in conjunction with lipopolysaccharide (aka LPS). This may be one reason why there is additional negative impact when there is a co-challenge with T-2 and gram-negative bacteria. Control of other dietary mycotoxins or LPS concurrently with T-2 may be important in any attempt to decrease its effects.
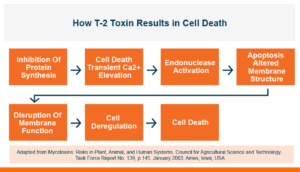
T-2 toxin decreases the productivity of poultry and livestock by inhibiting protein synthesis at the cellular level and causing cell death. In eukaryotic cell’s DNA, RNA, and protein, synthesis is inhibited by T-2 toxin. It also induces apoptosis or programmed cell death.
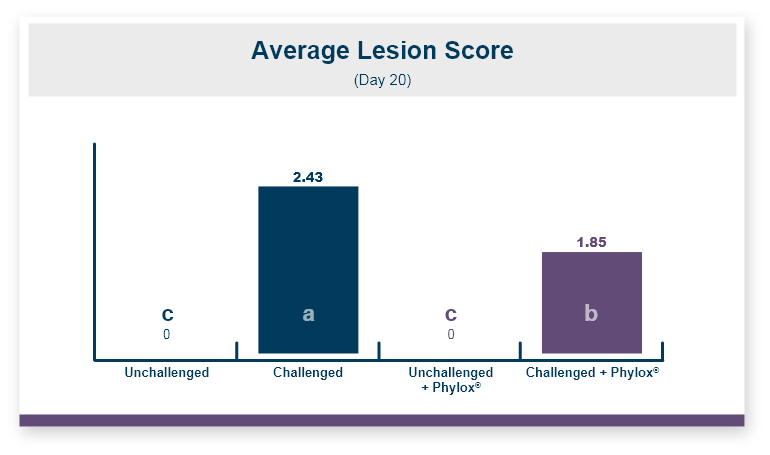
A major concern in poultry is how T-2 affects the gastrointestinal tract starting with lesions of the beak and gizzard and going through the entire gut. These lesions will affect feed intake, gain, and feed efficiency. But T-2 can affect all aspects of production and reproduction, so egg production and hatchability also need to be considered. In early research looking at the effects of T-2 on hatchability, 2 ppm of T-2 toxin was fed to laying hens, egg production decreased by 3.8 percent, fertility of the eggs that were laid decreased by 1.7 percent, and hatchability of fertile eggs decreased by 5.6 percent. This is a substantial loss of hatched chicks because of the toxin in the feed.
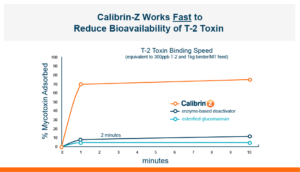
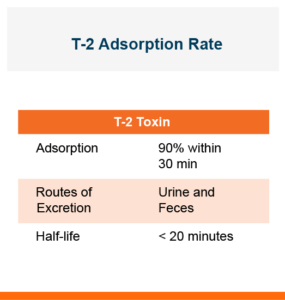
T-2 is quickly adsorbed. And it can be adsorbed through the lungs, the skin, or through the gastrointestinal tract when ingested in the feed. Approximately 90% of T-2 is adsorbed into the body within 30 minutes of ingestion, but it does have a short half-life of less than 20 minutes. T-2 producing Fusarium molds can occur in feedstuffs either during a warm and moist growing season or during storage under high moisture, especially if stored grains have damage such as broken or cracked kernels. The best option for producers is to use feedstuffs free of all toxins, however, the reality is that this is not always possible. In those cases where feedstuffs are being fed that may contain T-2 it would be beneficial to have a fast-acting toxin binder in the diets.
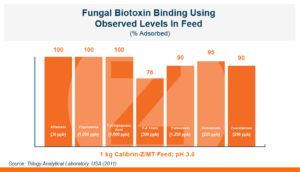
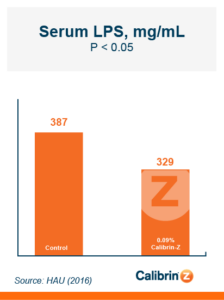
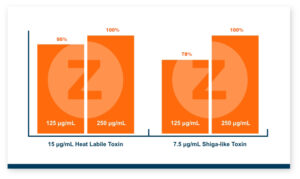
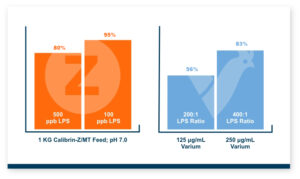
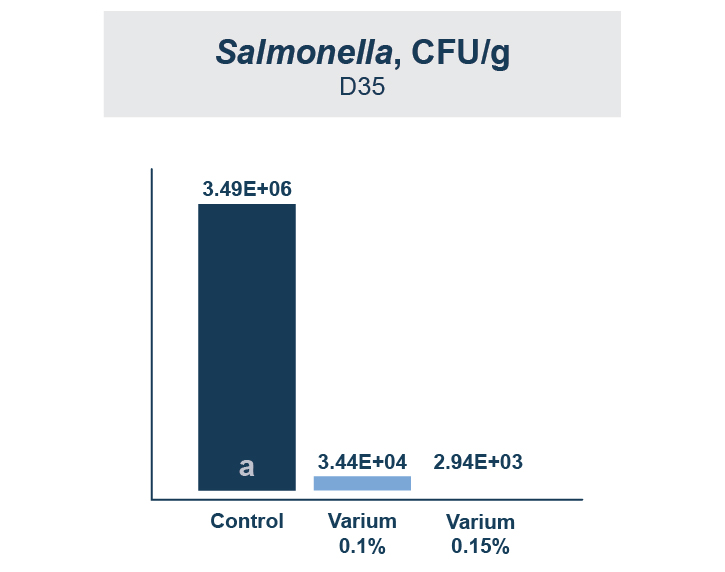
Because T-2 is so damaging and so rapidly absorbed, the toxin binder that is used needs to work and work fast. Calibrin®–Z, available in select international markets, adsorbed ~70% of T-2 toxin within 1 minute in research looking at speed-of-binding in vitro. This was approximately 24 times faster than the other products used in the trial. Additionally, Calibrin-Z had previously been shown to bind other mycotoxins and LPS in vitro and in vivo, which may be important during a T-2 challenge. A test to determine the binding ability of Calibrin-Z in vitro was conducted to look at seven common fungal biotoxins where the binder-to-toxin ratio was as if there was 1 kg of Calibrin-Z per metric ton of feed vs. observed concentrations of mycotoxins in feed. In vitro data showed that Calibrin-Z could bind LPS, but it has also been seen in vivo when Calibrin-Z was being fed to laying hens.
Calibrin-Z Mitigates the Effects of T-2 Toxin in Broiler Chicks
Recently, research was conducted at a large university in Brazil to determine the effects of Calibrin-Z on broiler chickens challenged with dietary T-2 Toxin. For this experiment, a total of 180 one-day-old male Cobb 500 broiler chicks were used. At the beginning of the trial the birds had an average body weight of 47 grams, with the average initial weight for each bird being equal. They were fed three different treatments 1) Unchallenged Control; 2) Challenged Control with 2 ppm T-2 Toxin; and 3) 2 ppm T-2 Toxin with 0.5% dietary Calibrin-Z. They were fed the treatment diets for 21 days. No aflatoxins, deoxynivalenol, diacetoxyscirpenol, fumonisins, ochratoxin A, T- 2 Toxin or zearalenone were detected in the feed ingredients that were tested before mixing the diets. The T-2 Toxin that was added to the feed for the challenged treatments was produced by Fusarium sporotrichioides fungi, and was 82% T-2 Toxin, 18% HT-2 Toxin. There were 6 pens that were randomly assigned to each treatment and there were 10 chicks in each pen. Chicks had free access to a constant supply of food and water. The diet was corn-soybean meal-based and formulated according to requirements in the Cobb Broiler Management Guide.
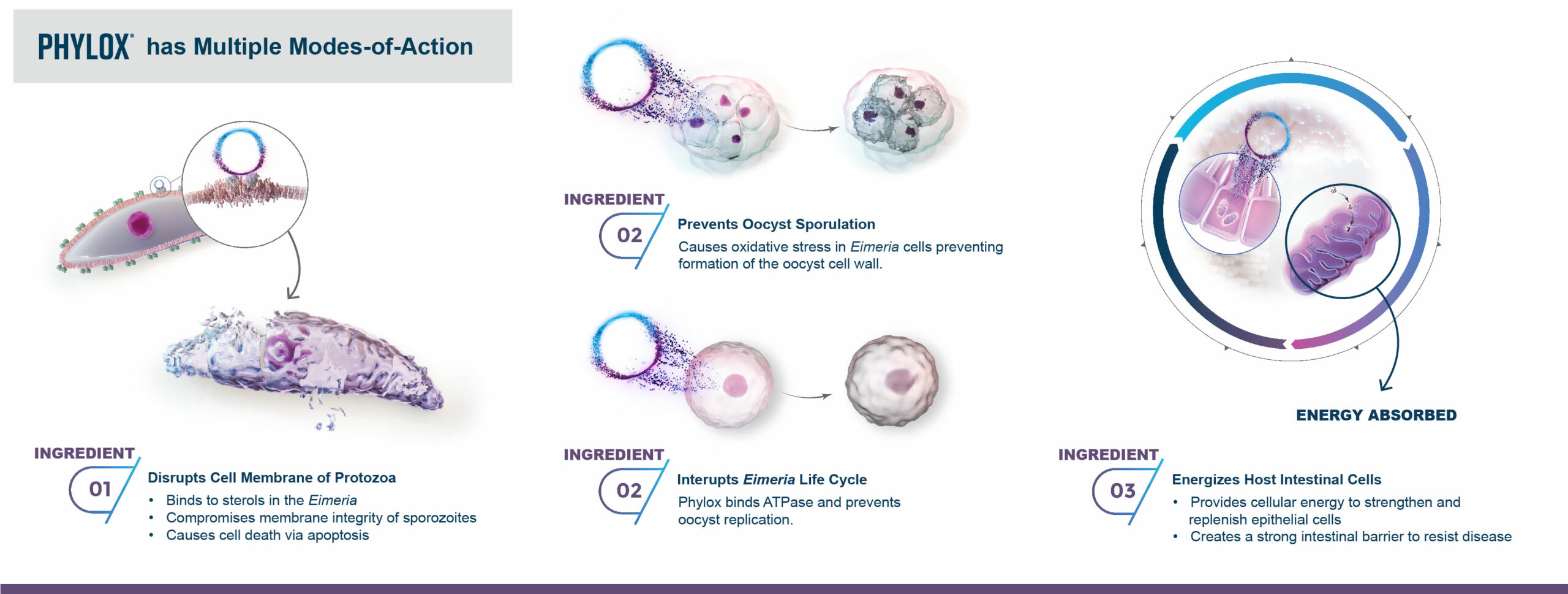
The intent of the study was to determine the effect of T-2 toxin on growth performance of broilers and how the addition of Calibrin-Z helped to mitigate any negative effects. Calibrin-Z is a unique calcium montmorillonite that has been shown to bind toxins, both fungal and bacterial, as well as lipopolysaccharides (LPS).
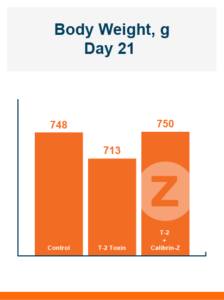
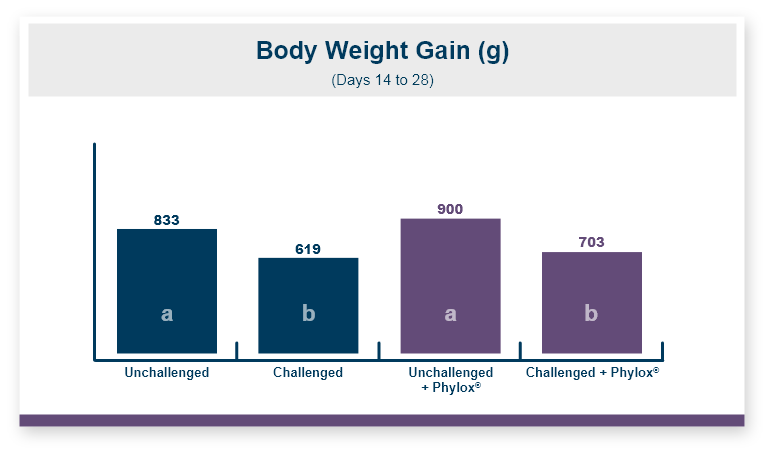
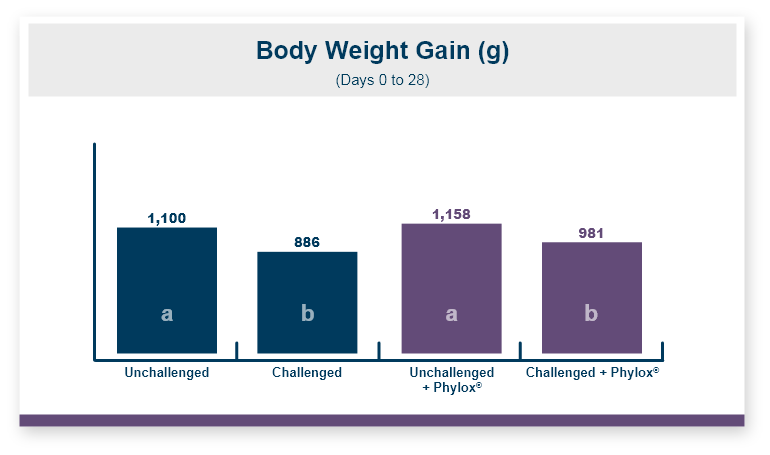
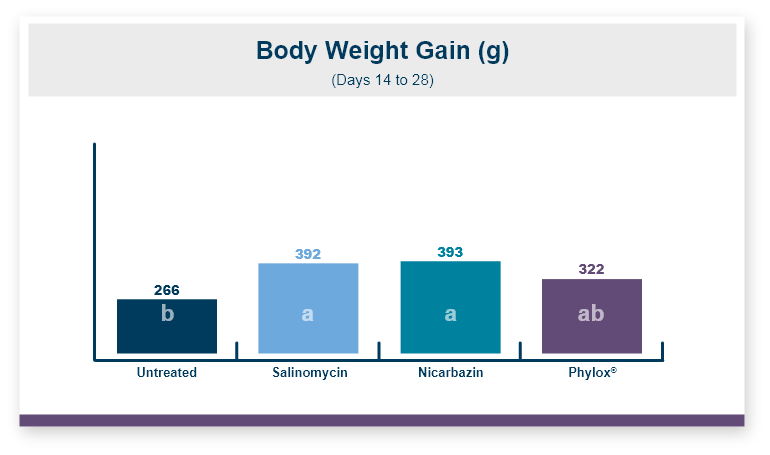
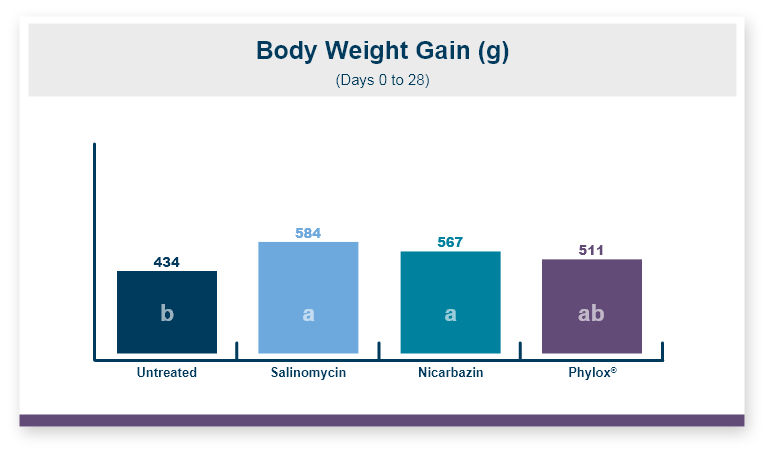
Feeding Calibrin-Z to the birds challenged with T-2 toxin increased body weight by 5% compared to the birds that were fed diets with T-2 toxin and no Calibrin-Z. This improvement returned body weight to that of the unchallenged control birds. In this experiment, there was no effect of feeding T-2 on feed intake with birds on all three treatments having equal feed intake.
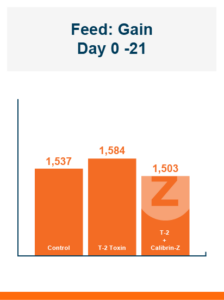
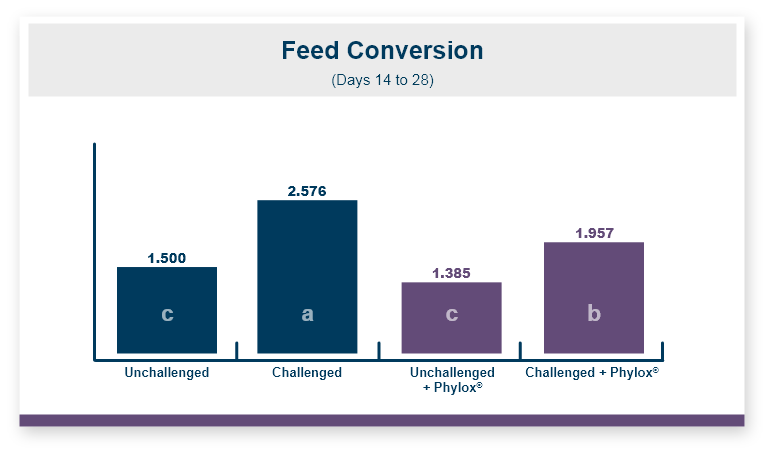
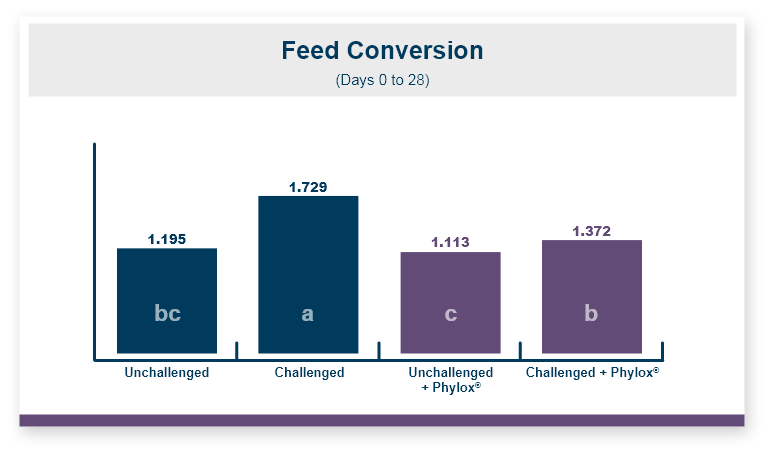
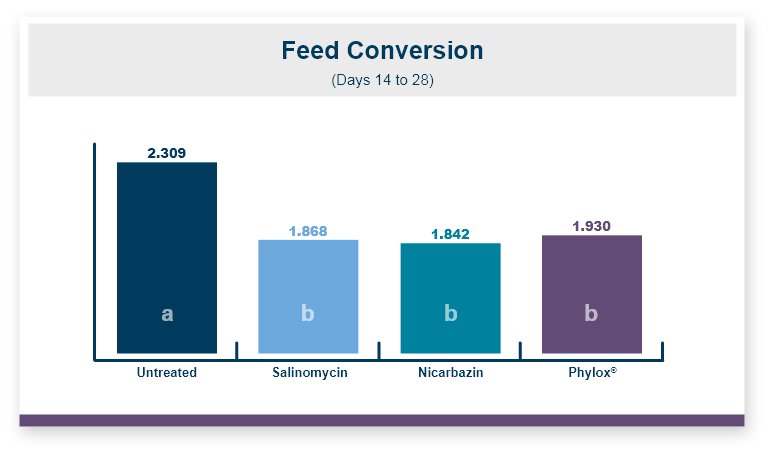
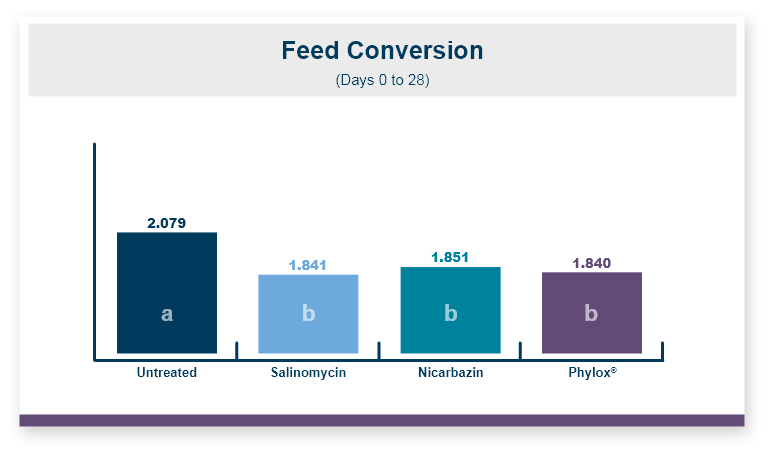
Because there was no difference in feed intake the feed conversion ratio followed the same pattern seen in body weight. Feeding Calibrin-Z to birds challenged with T-2 toxin improved feed conversion by 8 points, with values of 1.50 for Calibrin-Z fed birds compared to 1.58 for birds that only received T-2 toxin in the feed.
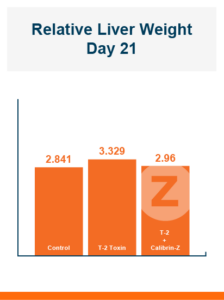
While aflatoxin is the mycotoxin best known for its negative effects on the liver, T-2 toxin can also have bad effects. In this study, relative liver weight was higher in the challenged control, 3.33%, compared to the unchallenged control, 2.84%, but feeding Calibrin-Z again mitigated the negative effect of the T-2 toxin, with challenged birds fed Calibrin-Z having a relative liver weight of 2.96%.
T-2 Toxin is a fast-acting mycotoxin that has a tremendous impact on animal performance. It acts in a synergistic way with challenges from other mycotoxins and lipopolysaccharide. To decrease its impact, you need a fast-acting toxin binder to control T-2 quickly while also controlling other potential problems. Calibrin-Z is a fast-acting multi-toxin binder that has proven results.
As the animal health business of Oil-Dri® Corporation of America, Amlan products are backed by Oil-Dri’s 80-plus years of mineral science expertise. Oil-Dri and Amlan are vertically integrated and own every step of the production process to consistently deliver safe, high-quality animal health products around the world. Calibrin-Z, a calcium montmorillonite clay, is sold as a broad-spectrum toxin binder. To understand how Calibrin-Z can work in your production system, contact your local Amlan representative.
References:
Chi, M. S., C. J. Mirocha, H. J. Kurtz, G. Weaver, F. Bates, and W. Shimoda. 1977. Effects of T-2 Toxin on Reproductive Performance and Health of Laying Hens. Poultry Sci. 56:628 – 637.
Tai, J.-H. and J. J. Pestka. 1988. Synergistic interaction between the trichothecene T-2 toxin and Salmonella typhimurium lipopolysaccharide in C3H/HeN and C3H/HeJ mice. Toxicol Lett 44:191–200.
Mycotoxins: Risks in Plant, Animal, and Human Systems. 2003. Task Force Report No. 139 Council for Agricultural Science and Technology. Ames, Iowa, USA.