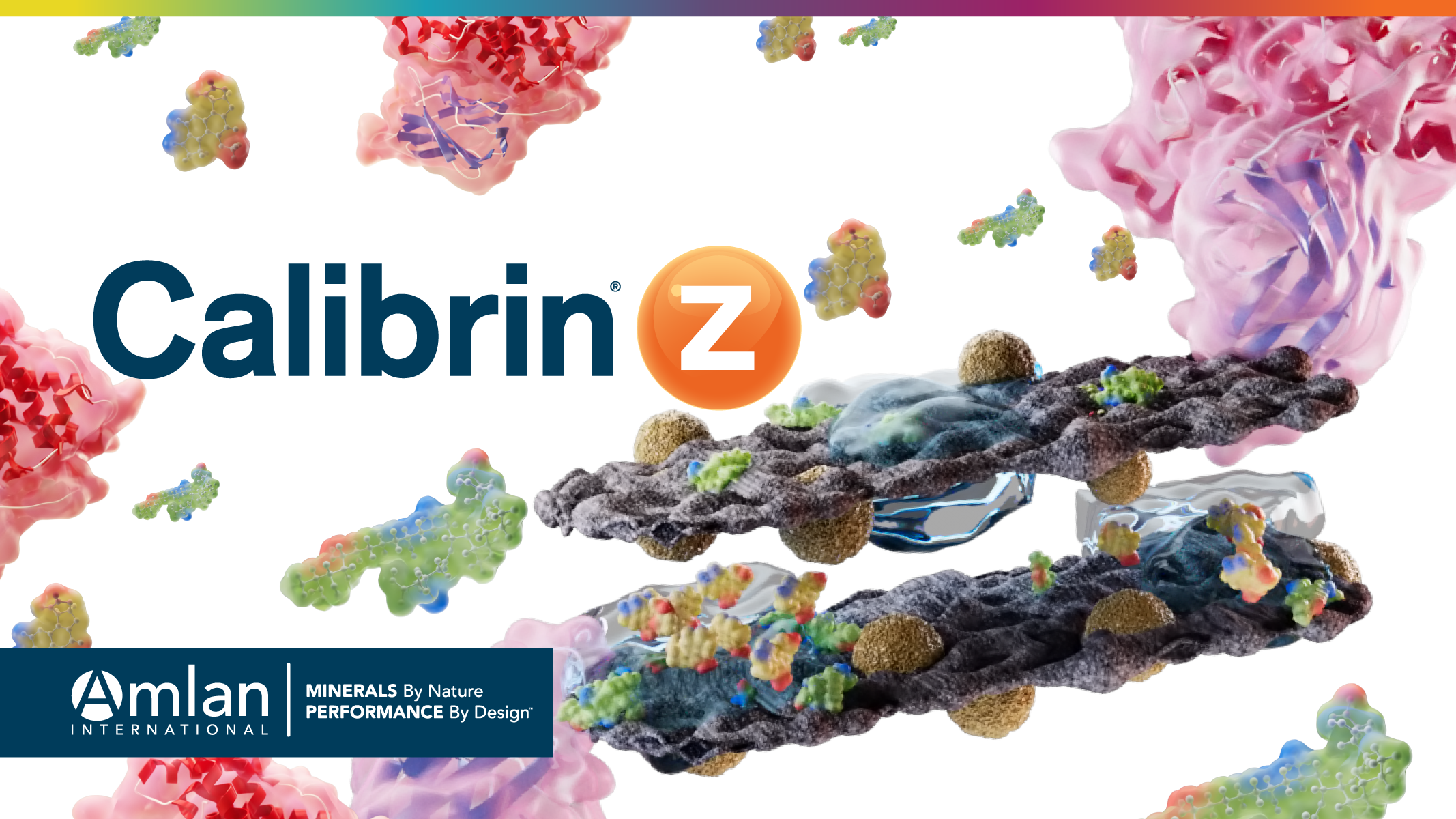
(Figure 1) Platinum octaethylporphyrin molecules seen adsorbed by Calibrin-Z using Cryogenic Transmission Electron Microscopy. Platinum (pink) seen under microscopy indicates where the organic compound was bound in the interconnected pores of the unique clay mineral.
Providing Economic Value for More Than a Decade
For more than 16 years, Calibrin®-Z (available in select international markets) has helped poultry producers mitigate the damage that mycotoxins cause to their livestock, their sustainability, and their bottom line. Calibrin-Z’s ability to bind mycotoxins, fungal toxins that negatively affect the health and performance of livestock, has been shown both in experimental settings and on the farm. The implication from this research was that the unique clay mineral that comprises Calibrin-Z was blocking the negative effects of mycotoxins on animal performance by adsorbing the toxins in the pores of the clay. The way the binding occurred was known but had not been seen directly.
Until Now!
Scientists from Oil-Dri worked with university scientists to enable us to see organic molecules binding to Calibrin-Z (Figure 1). To do this they used Cryogenic Transmission Electron Microscopy. This type of microscopy is used to look at biological and materials structures at an almost atomic level. The material of interest is flash-frozen to keep from damaging the structure of the organic material that is being observed.
Octaethylporphyrin, is an organic molecule that was chosen to represent the mycotoxins that Calibrin-Z normally adsorbs. It has a general size and planar orientation similar to that of mycotoxins. Platinum is not an element that is typically found in the clay mineral that makes up Calibrin-Z and can be seen using cryogenic transmission electron microscopy. This combination of factors makes it an excellent marker to use to visualize Calibrin-Z’s binding sites. When the organic portion of the platinum octaethylporphyrin molecule is adsorbed onto the Calibrin-Z binding sites you can see the platinum with a cryogenic transmission electron microscope. The platinum in the picture taken under the microscope was interspersed between the layers and on the outer surface of the Calibrin-Z particles. This shows that the organic compound was bound in the interconnected pores as was anticipated.
Selection and Quality
Calibrin-Z’s natural ability to adsorb biotoxins is based on the clay mineral used in its manufacture. The source of the clay mineral was chosen after years of testing and comparisons of a multitude of different potential sites. This source was selected based off its innate ability to bind toxins, the ability to improve that binding with processing, and its benign chemical profile. With vertical integration, mine to market traceability, and decades of reserves, this unique clay mineral is the foundation of Amlan International’s animal health products.
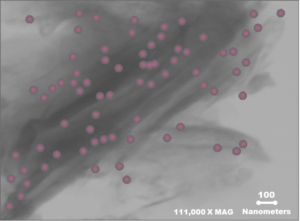
Calibrin-Z is composed mainly of calcium montmorillonite with opal lepispheres. The opal lepispheres are intimately interwoven within the nano-scale layers of montmorillonite. They help Calibrin-Z maintain its structure during a proprietary processing step that expands the number of biotoxins Calibrin-Z adsorbs. The unique structure of Calibrin-Z is vital to its toxin binding capacity.
Absorption and Adsorption
A kilogram of Calibrin -Z has approximately the same surface area as 60 soccer fields. This is because over 99% of Calibrin-Z’s total surface area is inside the particle. Calibrin-Z’s internal network of interconnected channels and pores is ~50% of its total volume. When Calibrin-Z is fed to livestock or poultry, fluid in the intestine rapidly absorbs into the mineral’s pores through capillary action. Biotoxins in the fluid move inside via the networks of capillary channels. From a molecular perspective it is as if they are traveling on a superhighway. Biotoxins adsorb once they reach the binding sites on the pores’ surfaces.
The biotoxin molecules are attracted onto the pore surfaces via adsorption, this is both chemisorption and physisorption. Biotoxins will structurally coordinate themselves onto charged surfaces and bind via ion-dipole and electrostatic interactions. While mycotoxins tend to be smaller and can enter the pores of Calibrin-Z and bind there, bacterial toxins tend to be larger but may also bind. Theoretically, there are special physical properties that allow the molecular conformation of the bacterial toxin to become distorted, which allows them to adsorb onto macro-surfaces within the pore spaces. Someday we may be able to use microscopy to see that, too.

Because of its structure, the clay mineral that Calibrin-Z is made from is naturally hydrophilic and will bind to polar molecules. But Calibrin-Z undergoes a proprietary processing method that causes dehydroxylation of the clay mineral’s crystal structure. During this process the opal lepispheres spread between the layers maintain its channels and binding sites. Thus, Calibrin-Z continues to bind polar molecules, such as the mycotoxin aflatoxin, but processing also allows it to have the ability to bind non-polar mycotoxins such as zearalenone. The ability to mitigate the effects of multiple mycotoxins has been shown using both in vitro and in vivo research.
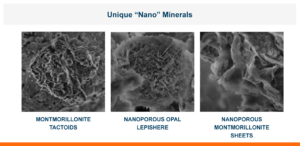
Because of Calibrin-Z’s proprietary heat treatment the toxins that it adorbs include a broad-spectrum of polar and non-polar toxins. Therefore, Calibrin-Z has shown high adsorption properties for mycotoxins, enterotoxins, and endotoxins.
Examples of Biotoxins Bound by Calibrin-Z

Natural and Reliable to Use
Calibrin-Z is shown to be a reliable and effective biotoxin binder. When added to animal feed at up to 5X the recommended dose it showed no negative effects. In fact, there was often a numerical improvement in gain, feed intake, or feed conversion when Calibrin-Z was added to an unchallenged diet. This indicates that there was no significant negative effect of Calibrin products on nutrient utilization.
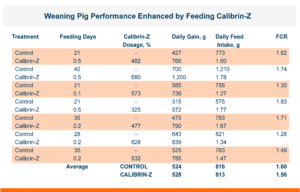
The unique surface chemistry and structural properties of the calcium montmorillonite in Calibrin-Z, added to its proprietary thermal-processing method, are what provide its optimal toxin binding capacity. This is what sets Calibrin-Z apart from other clay-based products. We have long known this because of its structure, how it works in vitro and how for more than a decade it has improved the performance of livestock and poultry. And now we, and you, are able to see it with our own eyes.
To learn more about broad spectrum biotoxin binder Calibrin-Z, and how you can add it to your poultry and livestock feed, visit amlan.com