Salmonellosis is a foodborne pathogen that causes illness and death worldwide. A blended feed additive has been shown to have good effects in vitro on a wide range of Gram-negative and Gram-positive bacteria and in vivo to mitigate the effects of Clostridium perfringens in broilers and E. coli in swine. Thus, it was decided to investigate the ability of the proprietary blend of essential oils, medium-chain fatty acids, and an activated toxin-adsorbing mineral (NeutraPath® available in select international markets) to control Salmonella. In vitro and in vivo research with Salmonella typhimurium and Salmonella heidelberg showed the blend could reduce prevalence and bacterial load of Salmonella in broiler chickens. Based on these in vitro and in vivo data, feeding this blend could be a potential new method to help control Salmonella in broiler chickens and aid in control of pathogens at the farm level.
A Foodborne Pathogen
Salmonella is a very common pathogenic bacteria that can be passed from animals to humans. The typical symptoms of salmonellosis in humans are diarrhea, fever, and stomach cramps, with the occasional vomiting. Generally, this is mild and doesn’t require medical intervention, but it can be deadly, especially in young children. Worldwide, Salmonella is one of four main causes of diarrheal diseases, with diarrheal disease being the 2nd leading cause of death in children under five. In the United States approximately 1,350,000,000 people are infected annually, ~26,500 people are hospitalized, and ~420 die each year. The very young, the very old, pregnant women, and people with compromised immune systems are generally affected the most.
According to the World Health Organization, Salmonella, a hardy bacteria, can survive in a dry environment for several weeks but several weeks turns into several months if it is in water.
Sometimes salmonellosis can result from coming in direct contact with animals that carry the bacteria, typically reptiles or birds. There are two species of Salmonella, bongori and enterica. Salmonella bongori is normally associated with cold-blooded animals but can infect humans. Selling tiny turtles (those with shells less than 4 inches long) has been prohibited in the U.S. since 1975 because of their association with salmonellosis in children. Salmonella enterica has more serovars with approximately 80 that can infect humans and animals. With an increase in the popularity of raising your own chickens the CDC (Centers for Disease Control) has issued repeated reminders about the safe handling of chickens, including a reminder “don’t kiss your chickens” because of serious outbreaks of salmonellosis, especially among children, linked to raising chickens in the backyard.


But eating or mishandling raw or undercooked contaminated food is the source of most cases of salmonellosis in humans. Live poultry often don’t show signs of carrying Salmonella even if their intestines contain the pathogenic bacteria. There are multiple ways that birds can be exposed to Salmonella. Exposure can be through contaminated feed, from wild birds or rodents, or from a contaminated barn. It is even possible that poultry may be contaminated before the egg that they hatched from was laid. Hens can have bacteria in the ovary or oviduct and the egg can be contaminated before the shell forms around the egg, meaning that even clean, washed eggs could be contaminated. The Poultry Industry has been working diligently to control this problem and this source of contamination of broiler chickens has declined in recent years.
Even though a majority of the foodborne illnesses due to Salmonella originate from non-poultry sources, twenty-three percent of the Salmonella outbreaks in the U.S. are linked to poultry consumption (16.8% from chicken, 6.6% from turkey) with another 6.3% coming from eggs. Salmonella in the intestine of poultry can lead to contamination of poultry meat during processing. Proper cooking will kill the bacteria, but improper handling may spread bacteria around the kitchen and raw vegetables, undercooked meat or uncooked foods containing eggs (i.e., cookie dough) may still be contaminated with live bacteria. Together the CDC, FDA (Food and Drug Administration), and USDA (United States Department of Agriculture) have a goal of reducing Salmonella illnesses by 25% by 2030. In order to do this, they need to decrease Salmonella infections from all products regulated by the Food Safety and Inspection Service division of the USDA by 25%. One way to help reach this goal is to help minimize the amount of Salmonella that comes into the processing plant making it less likely that contamination of poultry meat will occur.
Poultry producers need help to accomplish this goal. Research has shown that a proprietary blend of essential oils, medium-chain fatty acids, and an activated toxin-adsorbing mineral (NeutraPath®, Amlan International, available in select international markets) may be of assistance. The blend has anti-virulence effects because of its ability to bind quorum sensing molecules, exotoxins, and endotoxins associated with bacteria. It also has direct bacteriostatic/ bactericidal effects against both Gram-positive and Gram-negative bacteria. Its efficacy has been proven over years of in vitro and in vivo trials at multiple research sites, against multiple bacteria, in multiple animal species.
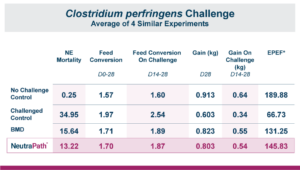
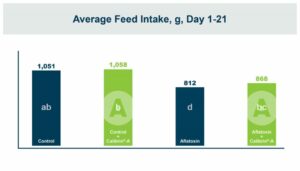
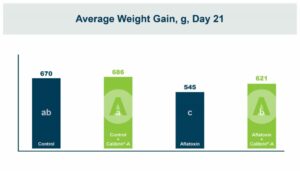
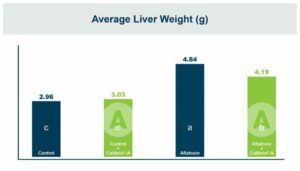
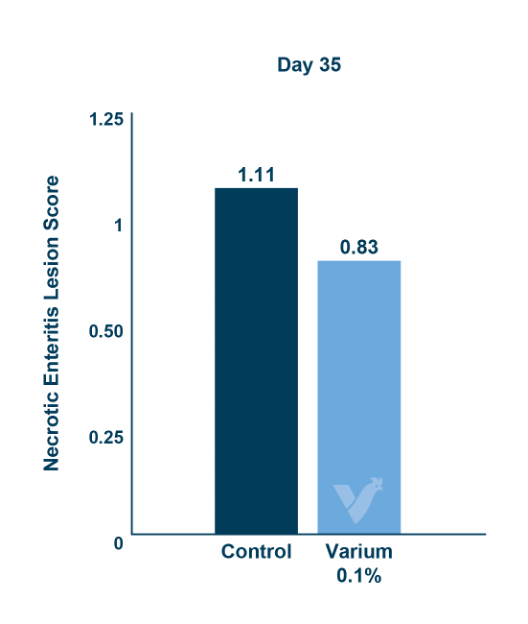
In vivo research in chickens showed that the blend decreased the effects of Clostridium perfringens. A summary of the studies showed that it improved mortality, gain, and feed conversion in challenged broilers. In weaning pigs, it was shown to decrease the impact of enterotoxigenic E-coli (F-18+). In the pigs challenged with E. coli the blend improved feed efficiency and decreased frequency of diarrhea. When the fecal microbiome was examined, there was a higher relative abundance of Lactobacillaceae and a lower relative abundance of Enterobacteriaceae. Enterobacteriaceae is a family of Gram-negative bacteria that includes both E. coli and Salmonella. These positive results led researchers to investigate its effects on Salmonella, with research being conducted both in vitro and in vivo.
Salmonella Research
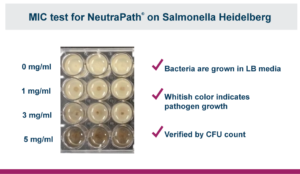
In vitro tests were used to determine the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of NeutraPath against S. heidelberg. Three different concentrations (1, 3 and 5 mg/ml) of NeutraPath with a control of 0 mg/ml were added to samples of an S. heidelberg strain. The MIC of NeutraPath for S. heidelberg was found to be 5 mg/ml. To determine the MBC Salmonella cultures were incubated at 37ºC for 16 hours without agitation. After incubation, bacterial counts were measured by serial dilution. A 30 μl aliquot of each dilution was plated onto lysogeny broth and incubated overnight. The MBC of NeutraPath for S. heidelberg was determined to be 4 μg/ml. These assays demonstrated that the blend has strong in vitro inhibitory and bactericidal activities against this key pathogen.
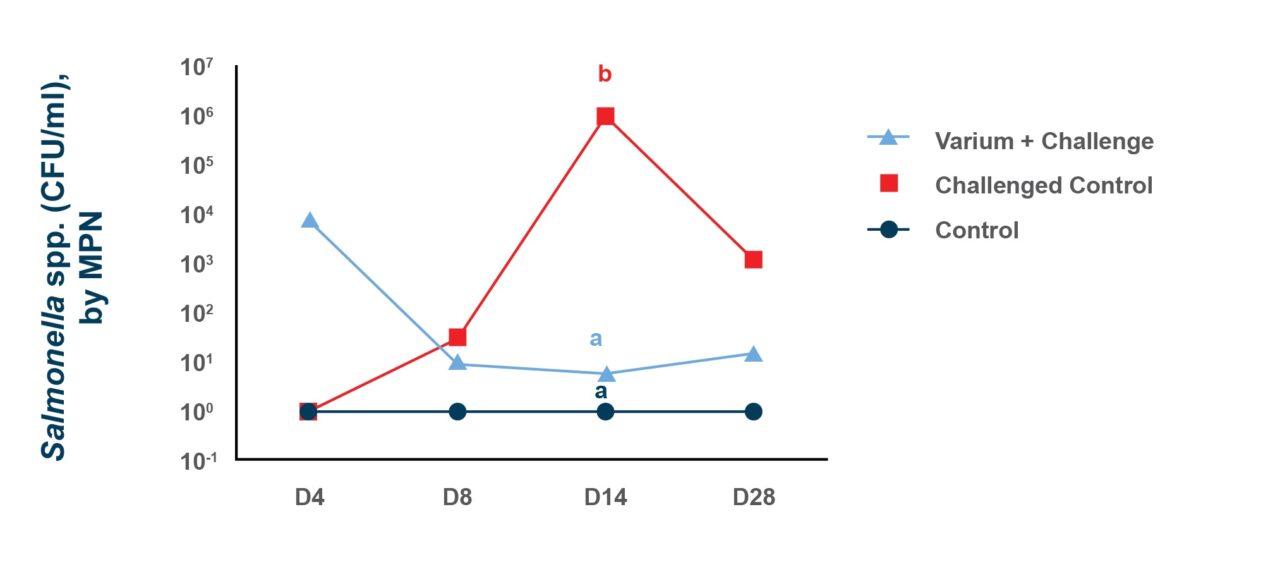
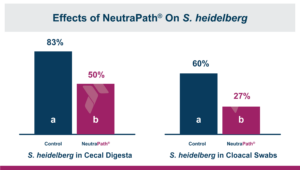
Because of the results seen in vitro researchers conducted an in vivo experiment. The researchers wanted to know how the blend would affect the Salmonella prevalence in birds previously challenged with S. heidelberg and fed for a short period of time before sampling.
After feeding the treatments for seven days, pre-moistened boot-sock swab sampling showed that there was S. heidelberg contamination in 100% of the pens. Cecal digesta samples and cloacal swabs were also collected from 10 of the broilers that had been directly challenged at hatch to determine Salmonella prevalence. The prevalence of Salmonella decreased by 40% in the cecal digesta (83.3% vs. 50.0%) and by 55% (60.0% vs. 26.7%) in the cloacal swabs when the blend was added to the diet for 7 days prior to testing.
Comparable studies were done at second location with another group of researchers to establish that the results from the initial studies could be repeated in other Salmonella serovars. This time studies looked at the effects of the blend on Salmonella typhimurium.
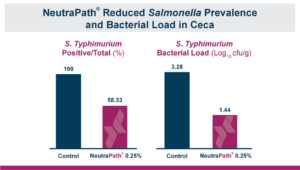
First an in vitro study digestion was used to simulate the crop, proventriculus and intestinal section of the gastrointestinal tract. Each “section” had pH and enzymatic conditions that would correspond to that area of the gut. Adding the antimicrobial blend inhibited the growth of S. typhimurium, reducing the total colony forming units recovered in each section. This positive result meant that an in vivo study was warranted. This time the broilers were started at one day of age. Thirty male broiler chicks were placed on two treatments. The treatments were a challenged control with non-treated feed, or birds fed that diet + 0.25% of the blend. At nine days-of-age chickens were given an oral dose of 106 CFU (Colony Forming Units) of live S. typhimurium. Twenty-four hours after the challenge ceca and cecal tonsils were removed so that they could be evaluated for Salmonella recovery. Both the number of positive samples and the amount of Salmonella bacteria found in those positive samples decreased in the treated birds. Feeding the blend decreased the ceca that tested positive for S. typhimurium by 41.7%, 100% of the tested ceca were positive in birds fed the untreated control compared to only 58.3% of the ceca in the birds that were fed the blend. In the birds that tested positive the total S. typhimurium bacterial load recovered in the ceca also dropped by 1.84 log10 CFU/g compared to the untreated control.
Conclusion
Around the world foodborne salmonellosis continues to be a problem. Regulators are targeting aggressive goals for salmonella reduction in poultry meat. A proprietary blend of essential oils, medium-chain fatty acids, and an activated toxin-adsorbing mineral has been shown to work against a variety of bacteria. Research has shown efficacy against C. perfringens, E. coli, and S. typhimurium and S. heidelberg. Based on the current in vitro and in vivo research adding the blended product has the potential to reduce Salmonella colonization in broiler chickens. This is a viable option for use in poultry health programs including those controlling Salmonella contamination. To learn more about NeutraPath or to contact a local representative, visit amlan.com.