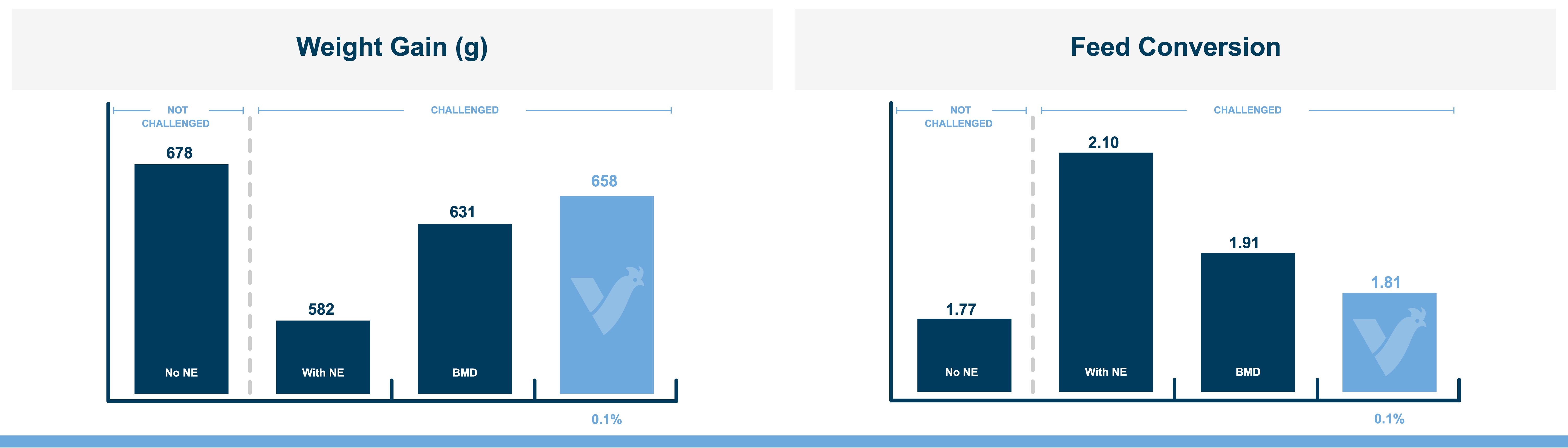
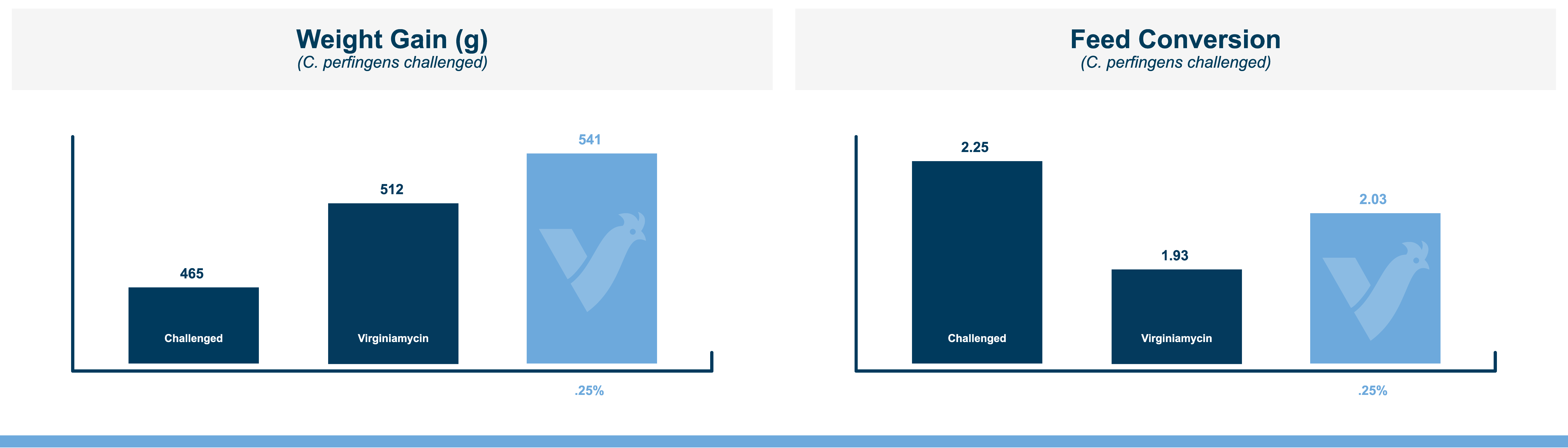
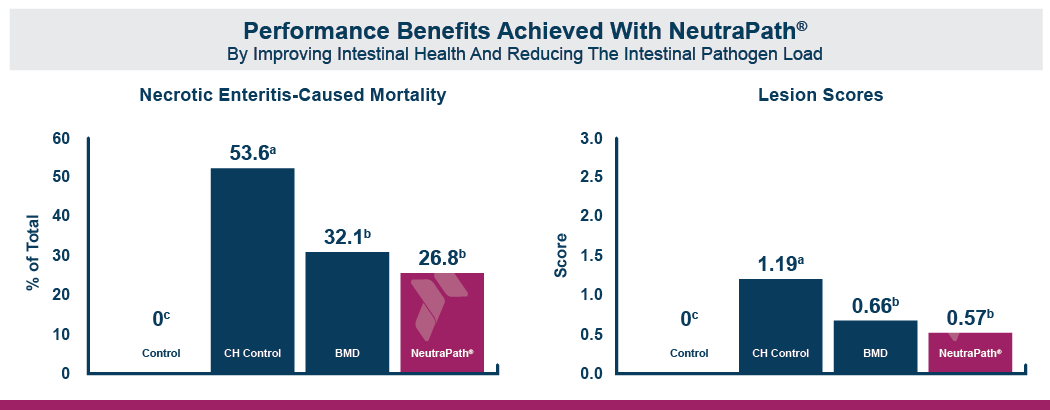
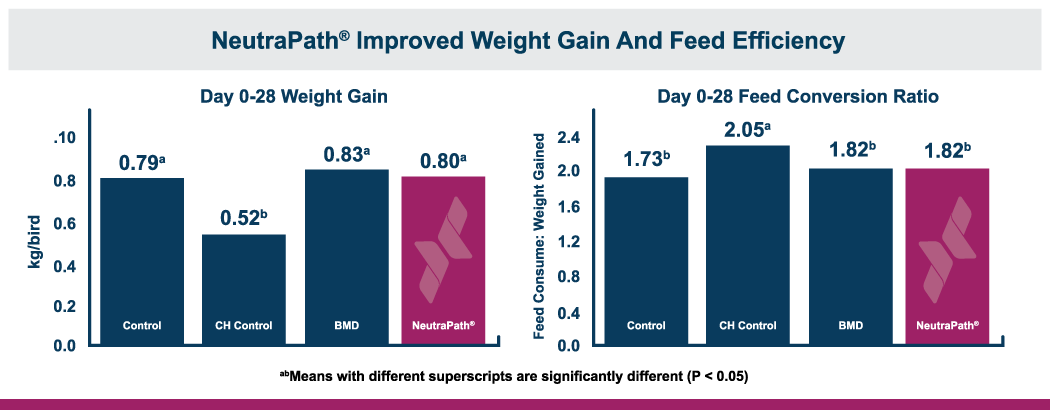
The fast onset of necrotic enteritis and the devastating production losses it inflicts make it one of the most challenging diseases for the poultry industry, particularly for antibiotic-free producers. Clostridium perfringens, the cause of necrotic enteritis, possesses a number of virulence factors that allow it to mount a fast, efficient attack on the host including potent toxins and cell-to-cell communication (quorum sensing). However, natural mineral-based products that can disrupt quorum sensing and reduce the virulence of C. perfringens are available.
The Pathogenesis of Necrotic Enteritis
C. perfringens is an anerobic, spore-forming pathogen found in the normal microbiota of poultry, as well as the poultry house. Necrotic enteritis occurs when predisposing conditions, such as a change in diet, immune status or intestinal pathophysiology, promote an overgrowth of C. perfringens. Coccidiosis can also increase the incidence of necrotic enteritis, as the damage that Eimeria spp. cause to intestinal epithelial cells promotes the invasion of C. perfringens (as well as other pathogens).
C. perfringens’ Rapid Infection Rate
C. perfringens is one of the fastest growing bacterial pathogens. Under optimal conditions it can replicate every 8 to 10 minutes — outgrowing other resident bacteria to achieve intestinal colonization.1 As well as a rapid growth rate, C. perfringens infection involves multiple steps, which likely occur simultaneously, including colonization, replication, nutrient procurement, evasion of host immune defenses, host tissue damage and transmission.2
Exotoxin Roles in Necrotic Enteritis Development
Multiple exotoxins can be produced by C. perfringens, including alpha-toxin and necrotic enteritis toxin B-like toxin (NetB). Alpha-toxin is cytotoxic to endothelial cells, red blood cells, white blood cells and platelets, while NetB toxin forms pores in cell membranes that allow electrolytes to rupture cells, causing cell death and necrotic lesions in the small intestinal mucosa.3 These two toxins are known to have a role in necrotic enteritis development.
C. perfringens Growth Depends on Host Nutrients
C. perfringens relies on nutrients from the host to live and multiply — a process which results in the destruction of host tissues (formation of necrotic lesions). C. perfringens lacks enzymes needed for amino acid biosynthesis and subsequent protein synthesis, so enzymes and toxins are released to degrade structural proteins from the host.4 The host amino acids and/or peptides are then taken in by C. perfringens for use in its own protein synthesis. 4
To produce energy, C. perfringens degrades large sugar compounds from the host and ferments them, producing gas that enhances the anerobic environment.4 C. perfringens also produces hyaluronidases that increase connective tissue permeability and help C. perfringens spread into deeper tissues.4
Quorum Sensing Controls Exotoxin and Enzyme Production
C. perfringens uses quorum sensing (cell-to-cell communication) to coordinate exotoxin and enzyme production to occur when its population reaches a density that supports the most efficient use of its metabolic resources.5 For example, to determine the optimum time to start producing NetB, the accessory gene regulator-like (Agr-like) quorum-sensing system sends out signals that are recognized by the VirR/VirS two-component regulatory system.5 Once the VirR/VirS system detects that the C. perfringens population has reached the threshold density, it switches on the expression of NetB and other virulence and related metabolism genes.2
Quorum Quenching Reduces Pathogen Virulence
Quorum quenching is an approach that can disrupt the quorum-sensing system of pathogenic bacteria, preventing cell-to-cell communication and the expression of quorum-sensing-controlled genes that produce toxins and other virulence factors. Additionally, quorum-quenching products should reduce the chance of antibiotic resistance, since they are modifying bacteria behavior rather than killing them.
Natural Quorum-Sensing Control
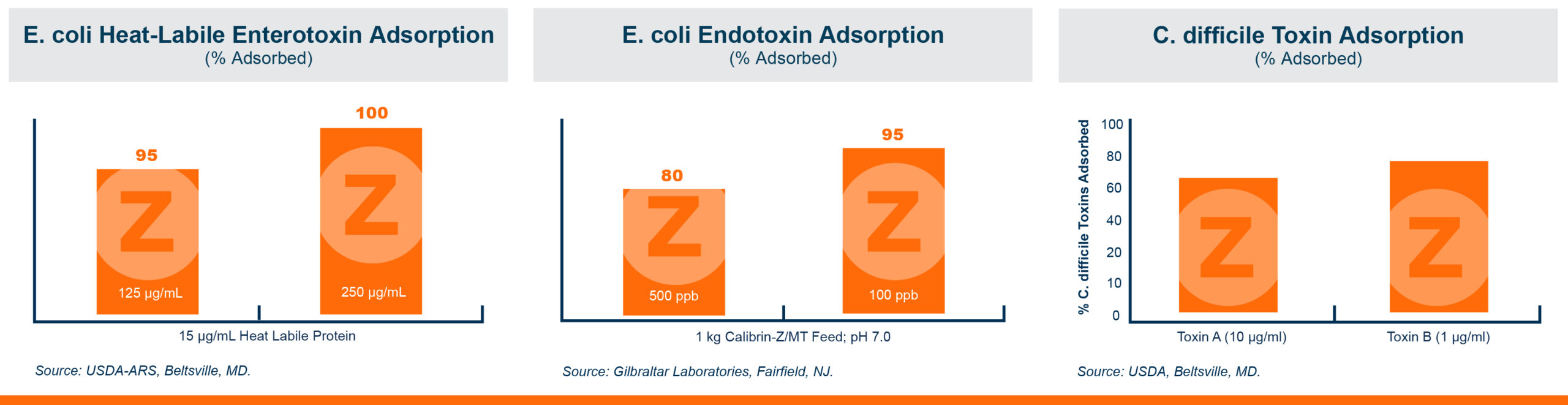
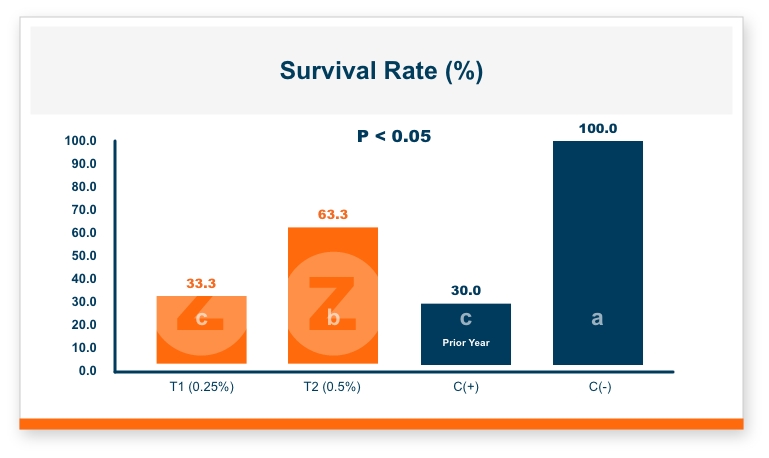
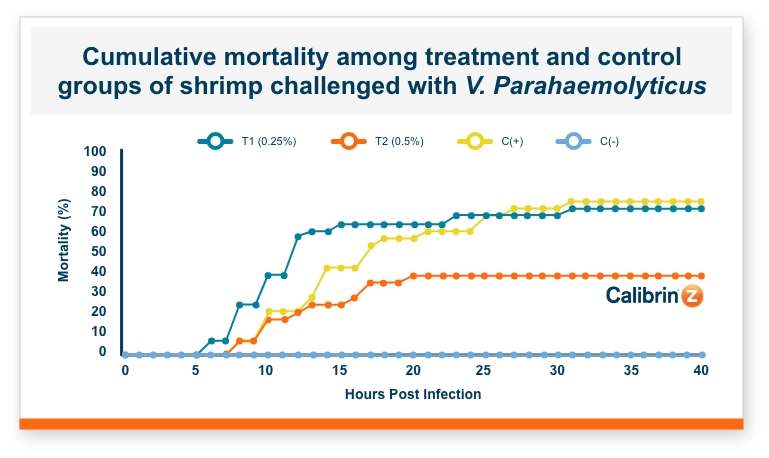
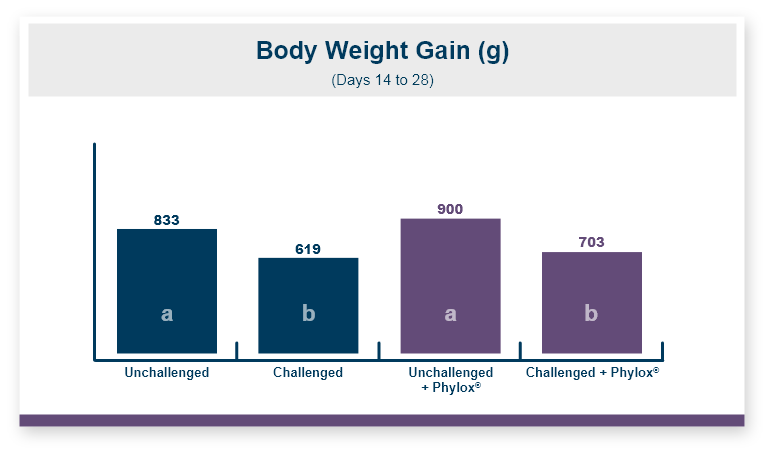
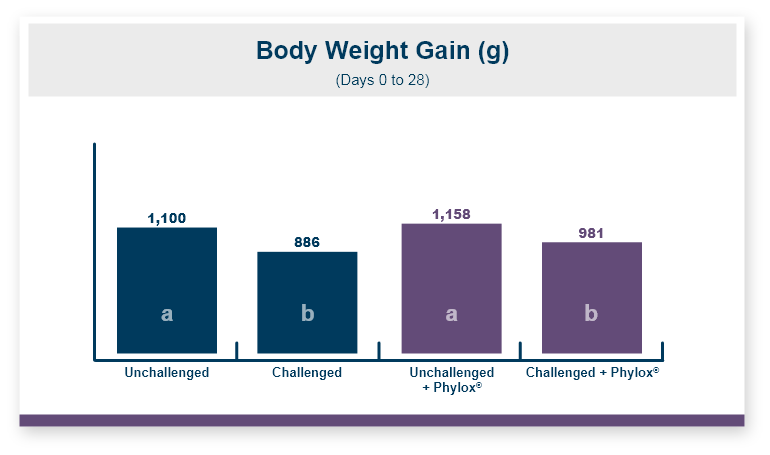
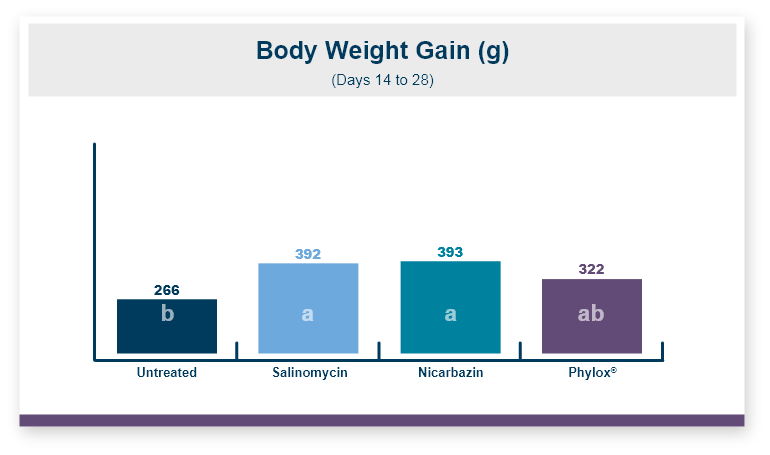
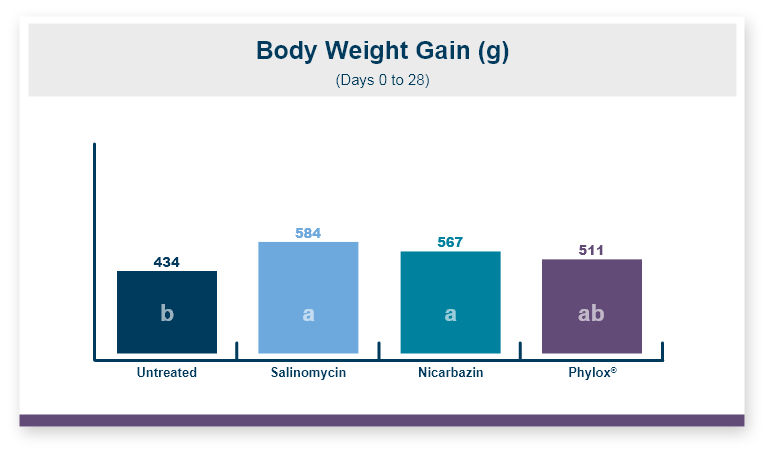
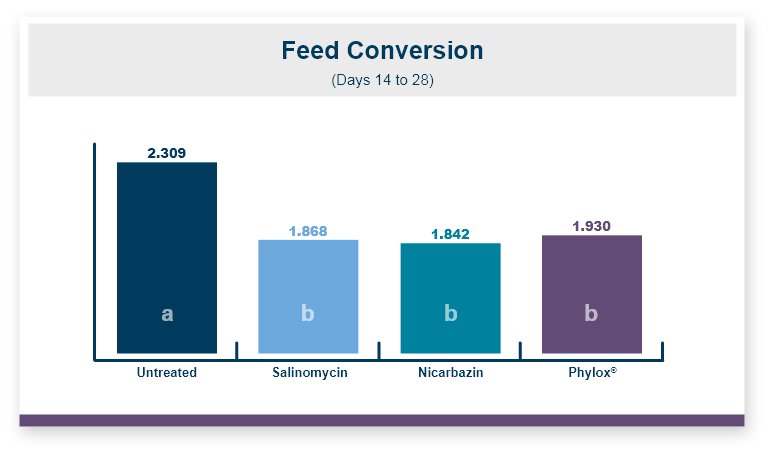
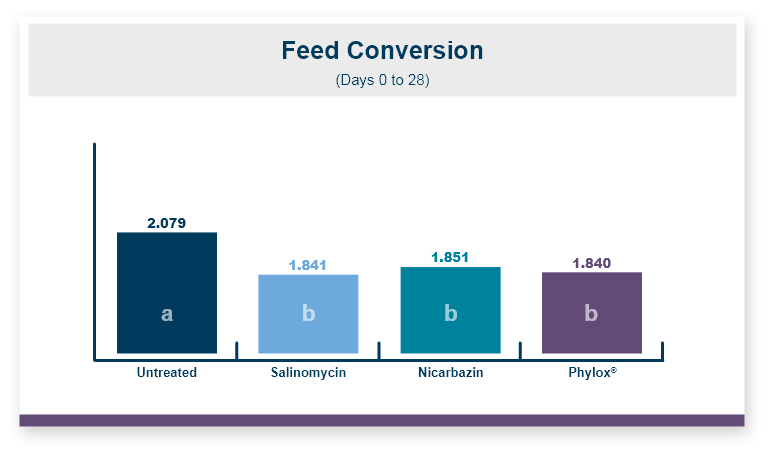
One product that has displayed quorum-quenching properties is the mineral-based feed additive Calibrin®-Z (available in select international markets). This all-natural single-ingredient product binds bacterial pathogens and the toxins they produce, as well as multiple mycotoxins, to help protect the intestinal barrier against enteric disease. Other natural mineral-based products can also help manage necrotic enteritis; read this article to learn more.
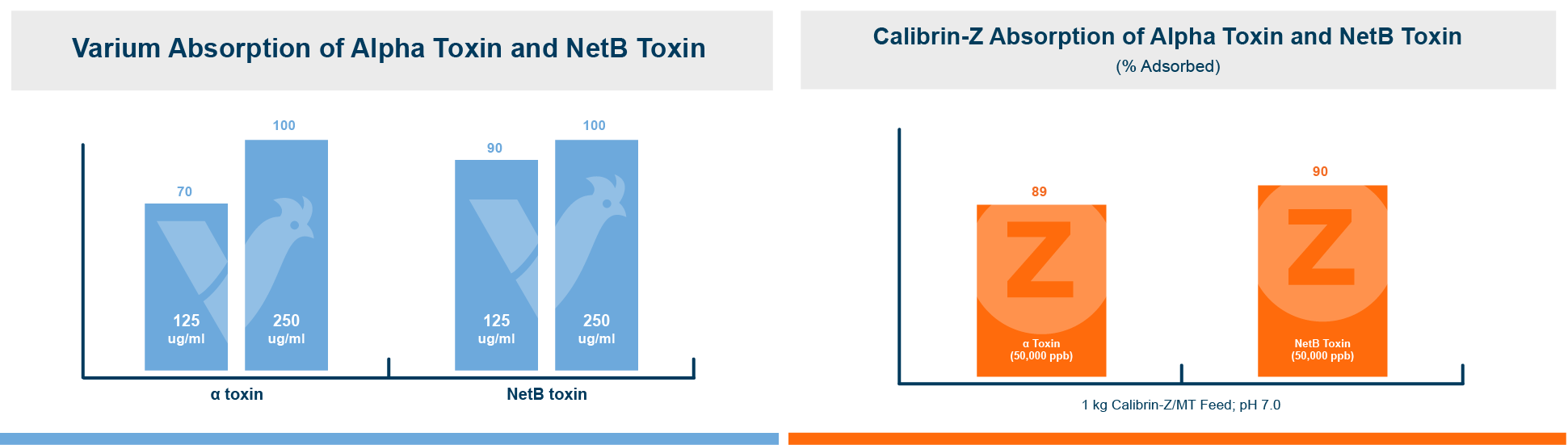
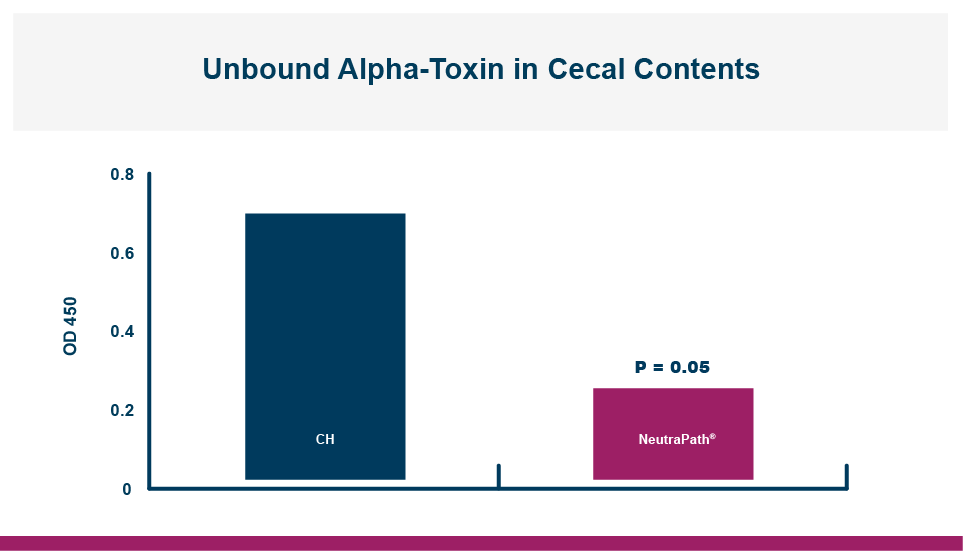
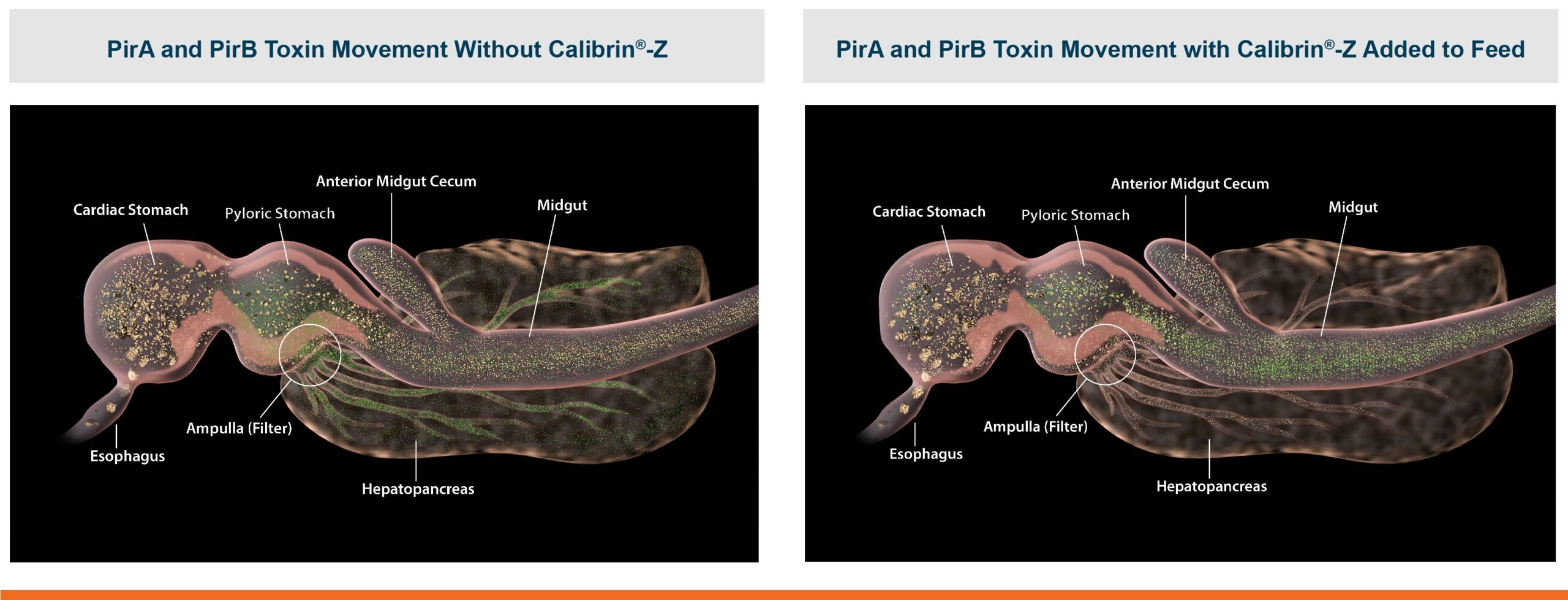
An in vitro study found that Calibrin-Z separated out quorum-sensing molecules by adsorption or catalytically broke them down into small fragments. By reducing the concentration of quorum-sensing biochemicals, Calibrin-Z can potentially disrupt the ability of pathogenic bacteria (including C. perfringens) to produce toxins, since quorum sensing controls this function. Calibrin-Z was also shown to effectively bind alpha-toxin and NetB toxin, further reducing the virulence of C. perfringens.
The global reduction in the use of in-feed antibiotics has compelled producers to rely on other management methods to maintain a healthy intestinal environment in poultry and reduce the risk of necrotic enteritis. The use of best-practice management strategies and inclusion of mineral-based feed additives that reduce the virulence of C. perfringens can assist in promoting intestinal health and maximizing production efficiency. To learn more about necrotic enteritis and natural mineral-based methods to control it, contact your local Amlan representative.
References
- Kiu R, Hall LJ. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg Microbes Infect. 2018;7:141.
- Prescott JF, Parreira VR, Mehdizadeh Gohari I, Lepp D, Gong J. The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know: a review. Avian Pathol. 2016;45:288–94.
- Chi, F. A Viable Adjunct or Alternative to Antibiotics: Meta-Analysis of Broiler Research Shows Natural Growth Promoter Delivers Feed Efficiency Equal to Antibiotics. Amlan International.
- Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S, Hayashi H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci U S A. 2002;99:996–1001.
- Yu Q, Lepp D, Mehdizadeh Gohari I, Wu T, Zhou H, Yin X, Yu H, Prescott JF, Nie SP, Xie MY, Gong J. The Agr-Like Quorum Sensing System Is Required for Pathogenesis of Necrotic Enteritis Caused by Clostridium perfringens in Poultry. Infect Immun. 2017;85:e00975-16.